Drug Trial Blog Reveals What It’s Like to be a Participant
ECD Global Alliance community member writes on his involvement in the dabrafenib and trametinib therapeutic trial at the National Institutes of Health
By Elizabeth Anderson, March 16, 2017
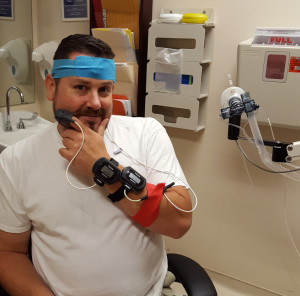

Left: Josef Lacy during his initial testing for the clinical trial at the National Institutes of Health. Right: Kevin O’Brien, NP, Josef Lacy, and Juvianee Estrada-Veras, MD (from left to right).
“[I] Started the day [by] stripping down to my skivvies and getting photographed from multiple angles, including close-ups of my hands, face, and eyes. I wasn’t allowed to tease my bangs, so it wasn’t a good glamour shots shoot. These pictures will be used to baseline my appearance as time goes on.” – Three Days a Blur (Josef Lacy, 2016)
Josef Lacy, Erdheim-Chester disease patient and ECD Global Alliance community member, humorously recounts the data collection phase of the National Institutes of Health’s (NIH) clinical trial in his personal blog Josef’s ECD Diary.
Participating in a drug trial or knowing what goes into testing a drug for efficacy and safety is a mystery to many. Demystifying the process, Lacy documents the journey of being diagnosed with an ultra-rare disease, his decision to join a phase 2 therapeutic trial, what it took to be eligible, and what happens once selected.
“This week has been amazing, intense, educational, more than one can imagine, scary, sobering, encouraging, enlightening, frustrating, and every adjective you can throw at it. I am told I have the chance to have a somewhat normal life. I’m taking that chance, if I get it, [I’m] running it like a pony at the Preakness” – Three Days a Blur (Josef Lacy, 2016)
The quippy portrayal of Lacy’s experience first kept friends and family informed of his condition and the experimental treatment path he chose to embark upon.
Now, he believes that his blog can help others. Patients affected by the perplexing and commonly misdiagnosed illness can intimately learn what goes into participating in a drug study.
“I believe it’s a way to give somebody a heads up on what [a drug trial] entails,” said Lacy. “I thought it would be a little bit easier. It’s been harder than I thought. It’s not easy, but for me, it’s worth it.”
Erdheim-Chester disease is considered a rare blood cancer. A diagnosis may take years with this illness. It is an ultra-rare condition and little known in the medical field. However, ECD-knowledgeable experts believe that the condition is under-diagnosed due to its complexity.
The patient advocacy group, the ECD Global Alliance, encourages research and clinical trials to better understand ECD and the discovery of effective treatments for sufferers.
Successful clinical trials are key to obtaining FDA-approved drugs for use in ECD. Currently there are no FDA-approved treatments for ECD, although there are some promising trials.
Participation in an ECD clinical trial allows Lacy, and other ECD-patients, access to new and promising treatments. In addition, their participation helps enable future ECD patients to have access to successful therapies.
Learn more about Lacy’s clinical trial experience at Josef’s ECD Diary, www.joseflacy.wordpress.com. Visit www.erdheim-chester.org/studies-trials to find out about ongoing research and clinical trials involving ECD patients.