Treatment Decisions
Deciding on the proper treatment for ECD involves collaboration between the patient and their doctors. Treatment of patients can vary based on the country and the practitioner’s experience. However, most clinicians rely on consensus recommendations published in Blood or by the National Comprehensive Cancer Network® (NCCN®). These recommendations state that treatment depends on disease burden, mutational status, drug availability, and practitioner experience.
Selecting a Treatment
Fortunately, ECD patients have treatment options, some of which have proven very effective for most patients. A few patients with asymptomatic disease not involving major organs may enter a watch-and-wait period with no treatment. However, treatment is generally recommended, especially for those with critical organ involvement, such as the heart or central nervous system.
Treatment vs. Cure
Although there are effective treatments for ECD, a cure is yet to be found. The best treatments available today are called targeted treatments and are usually successful in controlling and often shrinking ECD lesions. At present, these treatments normally must be continued indefinitely, or the disease will most likely begin to progress again. However, with successful treatment, ECD can often be controlled as more of a chronic disease.
Types of Treatments
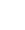
Typical ECD treatments fall into one of three categories: (1) targeted treatments, (2) chemotherapy, or (3) immunotherapy. It is universally established that targeted therapy should be offered to patients with neurological or cardiac disease when available in the patient’s country. In other circumstances, the following treatments are considered and/or recommended:
- Immune-modulating treatments
- Interferon
- mTOR inhibitor or sirolimus
- Cytokine inhibitor (Anakinra)
- Chemotherapy
These treatments should be discussed with the physician based on the patient’s symptoms and comorbidities.
Targeted Therapies
These are the newest and generally most promising treatments available for ECD. They typically halt the progression of ECD in most organs, although brain involvement must be watched very carefully. For some patients, it can be difficult for targeted treatments to cross the blood/brain barrier, reducing their effectiveness in halting ECD progression in the brain. Targeted treatments are expensive and may be challenging to obtain in some parts of the world.
Targeted treatments in ECD block the constitutive activation MAP kinases pathway cascade, addressing the various MAPK pathway mutations present in patients with ECD. The choice of targeted agent is often directed by the mutations present in the ECD tissue based on genetic testing.
- BRAF V600E Positive ECD
Approximately 50% of patients will test positive for the BRAF V600E mutation. Treatment options for these patients typically include a BRAF inhibitor, a MEK inhibitor, or a combination of both (in rare cases). Commonly used BRAF inhibitors include vemurafenib, dabrafenib, and encorafenib. Commonly used MEK inhibitors include cobimetinib, trametinib, binimetinib, or selumetinib. In the USA, vemurafenib and cobimetinib are FDA-approved treatments for ECD. - BRAF V600E negative ECD
Most patients who test negative for the BRAF V600E mutation will have a different mutation in what is called the MAPK pathway (also known as the Ras-Raf-MEK-ERK pathway). This pathway is a chain of proteins in the cell that communicates a signal from a receptor on the cell’s surface to the DNA in the cell’s nucleus. These signals control the cells’ proliferation, differentiation, development, inflammatory responses, and programmed cell death. MEK inhibitors are typically considered for all patients who do not test positive for BRAFV600E , including those patients where no mutation is found with any sequencing. Commonly used MEK inhibitors include cobimetinib, trametinib, binimetinib, or selumetinib. In the USA, cobimetinib is an FDA-approved treatment for ECD. - Rare Instances of a Rare Disease
Very infrequently, ECD patients may have other genetic abnormalities and do not respond to MEK inhibitors. Currently, the following mutations have been discovered in a very small percentage of ECD patients with limited reporting of the listed corresponding treatment.Discovered Mutation in the ECD Lesion Possible Therapy ALK fusion Crizotinib CSF1R mutation Pexidartinib NTRK gene fusion Larotrectinib NTRK gene fusion Entrectinib PIK3CA mutation Sirolimus RET fusion Selpercatinib - Other Targeted Treatments
Prior to understanding the genetic mutations seen in ECD, other targeted treatments were used, but without the dramatic good results often seen in the above treatments. Some earlier targeted treatments used for ECD included imatinib (tyrosine inhibitor) and sorafenib (kinase inhibitor). - Dosage of Targeted Treatments
It should be noted that most ECD patients can often only tolerate a low dosage of targeted treatment drugs. For this reason, the administered dosage prescribed for ECD patients is usually lower than that used in the treatment of other diseases. Currently, although these treatments must often be continued indefinitely, further dosage reductions are often possible once a patient’s disease is fully stabilized on treatment. Patients should always discuss with their doctor any proposed change in their treatment dosage before making a change. - Treatment Form
In most cases, all the targeted treatments are administered in pill form, once or twice daily. - Side Effects
The side effects of targeted treatments must be managed through a partnership between the patient and their medical team. Patients should always ask their medical team about possible side effects whenever beginning any treatment. The common side effects of BRAF inhibitors include rash, joint pains, and sun sensitivity of the skin. The common side effects of MEK inhibitors include rash, diarrhea, and swelling of the legs. Rare side effects of BRAF inhibitors include skin cancers or heart rhythm problems, and those of MEK inhibitors include vision disturbances and heart failure. - Treatment Cost
The cost of targeted treatment can sometimes be very high. In some countries, insurance and government payers will cover much of the cost of treatment. In some locations, there may be organizations that can help with the residual cost of treatment, depending on patient income. Yet, in other locations, access to targeted treatments is extremely limited. Patients should speak to their medical team about the costs of these treatments and discuss payment options. The ECDGA is available to work with patients and their medical teams to help search out payment options where possible.
Chemotherapy
Chemotherapy uses drugs that destroy cancer cells or other rapidly dividing cells. Multiple chemotherapy regimens have been used in the treatment of ECD. These include cladribine and methotrexate. Small studies have shown that some patients respond well to chemotherapy, although the response rate is much lower than that of targeted treatments. An advantage of these treatments is that some patients have experienced a prolonged response with a limited duration of treatment.
- Cladribine
Before targeted treatments were available, cladribine was used and studied as a treatment for ECD. It showed moderate efficacy for ECD patients. Typically, patients received cladribine for five days, every 28 days, for 2-3 months via intravenous injections. - Methotrexate
The use of methotrexate as a treatment for ECD has shown a low response rate. However, this treatment is normally well-tolerated and thus could be beneficial to those for whom it works. Methotrexate can be administered as an infusion, injection, or tablet. For ECD patients, it is normally administered as a weekly injection into the muscle.
Immunotherapy
Immune-modulating therapy can work by stimulating or suppressing the immune system to shrink ECD lesions.
- Interferon
The first treatment discovered for ECD was interferon, a form of immunotherapy. Where available, pegylated interferon alpha is the preferred interferon treatment because it is administered less often with a better tolerance profile. However, this formulation is not available in all countries. Interferon alpha-2a has been used in the treatment of ECD and needs to be taken more often. Interferon is normally administered at home via a shot under your skin or into a muscle. This treatment must most often continue indefinitely. Although some patients can easily tolerate interferon treatment, many patients report flu-like feelings when becoming acclimated to the treatment. This treatment has been found to slow the progression of ECD. - Sirolimus and Everolimus
Sirolimus and everolimus, forms of immunosuppressive therapy, were shown in a small study to have moderate efficacy for around 60% of ECD patients. This treatment is normally administered in tablet or liquid form once a day. - Biological Therapies
Biological therapy is a type of treatment that suppresses the abnormal chemicals (cytokines) produced by the ECD cells to cause inflammation, resulting in the shrinkage of lesions.- Anakinra
Small studies have shown that some patients have responded to Anakinra as a treatment option for ECD. Anakinra is usually administered as an injection under the skin. The study of anakinra as a stand-alone treatment for ECD has been limited but can be helpful for those patients with prevalent systemic symptoms. Anakinra has also been used to improve the tolerability of targeted treatments. - Other Biologic Treatments
Less often used biologic treatments include canakinumab, tocilizumab, and infliximab.
- Anakinra
Clinical Trials
Because of the rarity of this disease, clinical trials were not historically conducted to assess the effectiveness of treatments. Early documented treatments were based on case studies conducted with very small groups of patients, sometimes just a single patient. However, this is changing. Several studies and clinical trials are open to ECD patients in some countries today. When ECD patients enroll in a clinical trial, they can have access to some of the newest and best treatments available to ECD patients. All patients are encouraged to talk with their medical team about whether a clinical trial would be appropriate for their situation. Clinical trials allow patients access to the newest treatment options and pave the way for treatments to be scientifically proven effective in treating ECD. This has led to the FDA approval of two ECD treatments. FDA approval helps all ECD patients gain access to the treatment. There is strong hope that new treatments will be found in the not-too-distant future that will be easier to tolerate.
For more information, see:
- National Comprehensive Cancer Network (NCCN) Histiocytic Neoplasms, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology.
- Blood Journal article, https://ashpublications.org/blood/article/135/22/1929/452713/Erdheim-Chester-disease-consensus-recommendations
These publications have been authored by leading experts in diagnosing and treating ECD. If a patient is being seen by a doctor with little ECD awareness or knowledge, it is highly suggested that these documents be shared with them.
Last updated: November 15, 2024